How Many Electrons Fit in an Orbital
That means selenium keeps its electrons in four atomic orbitals and has six valence electrons or six electrons in its outermost orbital. Heat capacity is MUCH less than predicted.
How Many Electrons Can Fit In The First Energy Level At Level
Molecule 1794 extremely minute particle from French molécule 1678 from New Latin molecula diminutive of Latin moles mass barrier.
. To complete this picture leads us into the weird world of quantum mechanics. Electrons appeared to exhibit two types of motion in an atom. Instead the ion uses 6 orbitals from the 4s 4p and 4d levels to accept lone pairs from the water.
Arrow pointing to elements P 15 S 16 C l 17 A r 18 A s 33 S e 34 B r 35 and K r 36. Voltage dependence of photocurrent channels through a single molecule. The orbital diagram looks like.
These atoms are able to access the d-orbital. Classical views of the a orbital motion and b spin of an electron. The 8 electrons of Ni2 sit in five 3d orbitals.
Visualizing the densely packed nucleus in terms of orbits and shells seems much less plausible than the corresponding shell model for atomic electrons. Mechanism of photocurrent. According to Merriam-Webster and the Online Etymology Dictionary the word molecule derives from the Latin moles or small unit of mass.
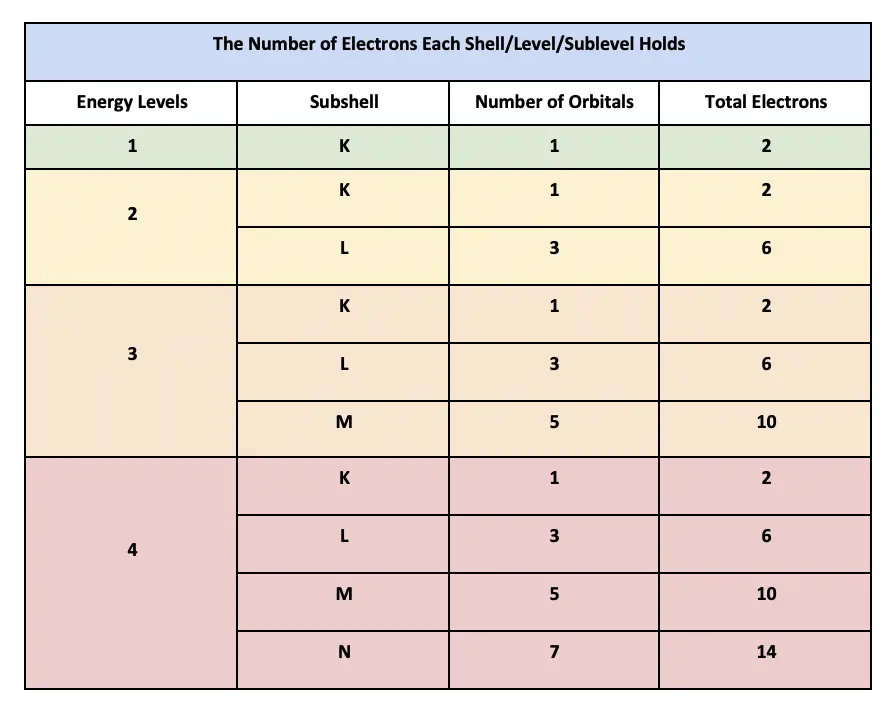
You can easily believe that an atomic electron can complete many orbits without running into anything but you expect protons and neutrons in a nucleus to be in a continuous process of collision with each other. An s-orbital holds 2 electrons. The 3d 4d etc can each hold ten electrons because they each have five orbitals and each orbital can hold two electrons 5210.
Electrons Discovered in 1897 Drude-Lorentz Model 1905-Electrons - classical particles free to move in a box Model. Then play a game to test your ideas. First we look at the n1 shell the first shell.
4s23d6---- 4s03d8 Need six orbitals for six ligands but. But same model predicted that all electrons contribute to heat capacity. All electrons contribute to conductivity.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Build an atom out of protons neutrons and electrons and see how the element charge and mass change. 4 3d orbitals are full only 1 3d orbital left Must hybridize 1 4s 3 4p and 2 4d to give.
This g orbital is not as commonly used as s p d and f because none of the elements we use needs a g orbital to have all of its electrons accounted for the highest known element is Oganesson which fills the 7p. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. The single electrons in the 3d level are NOT involved in the bonding in any way.
To unpair a set of electrons select an atom after all of its free electrons have been bonded. The motion of the electrons in the Rutherford model was unstable because according to classical mechanics and electromagnetic theory any charged particle moving on a curved path emits electromagnetic radiation. A vague meaning at first.
Thus to find the number of electrons possible per shell. Using quantum mechanics the behavior of an electron in a molecule is still described by a wave function Ψ analogous to the behavior in an atomJust like electrons around isolated atoms electrons. In 1913 Bohr proposed his quantized shell model of the atom see Bohr atomic model to explain how electrons can have stable orbits around the nucleus.
Atomic-scale measurement of photocurrent generation in a single molecule. The g orbital comes after the f orbital which only exists with excited states of the essential 118 known elements. Ni2 electron configuration 8 electrons.
Clearly Rutherfords model was missing something important and assumed something that cannot be true with regard to forces within the nucleus the orbital properties of electrons and the attractions between electrons and protons. So many questions and so few answers. When it forms an Fe 3 ion it loses the 4s electrons and one of the 3d electrons.
The vogue for the word used until the late 18th century only in Latin. In atomic physics the Bohr model or RutherfordBohr model presented by Niels Bohr and Ernest Rutherford in 1913 is a system consisting of a small dense nucleus surrounded by orbiting electronssimilar to the structure of the Solar System but with attraction provided by electrostatic forces in place of gravityIt came after the solar system Joseph Larmor model. Thus n1 shell can hold two electrons.
How many electrons can a G orbital hold. As atomic physics and chemistry began to explain the periodic table with the help of the Bohr model of the atom in the early 1900s magnetic properties were assigned to the electrons in atoms.

Krypton Orbital And Bonding Info Science Projects Teaching Chemistry Atom Model Project
No comments for "How Many Electrons Fit in an Orbital"
Post a Comment